$890.00 – $24,850.00
Maze Engineers provides the Rodent Auditory Brainstem Response Test.

MazeEngineers offers custom-built behavioral mazes at no extra cost—designed to fit your exact research needs. Eliminate reproducibility issues from poor sizing or lingering scent cues with precision-engineered, modular, and smart mazes that adapt in real time to animal behavior. Publish new protocols, run adaptive experiments, and push the boundaries of behavioral science.


Conduct ABR software |
Conduct ABR Maze software |
Maze Engineers Sound System |
Maze Engineers control box |
Speaker |
Real-time sound lever reader |
Electronic controller and sound generator |
Brain response recorder |
TDT Rz6 Multi-I/O high-frequency auditory processor |
Mouse Auditory Brainstem Response Test |
Isolation Cubicle |
Animal enclosure |
Maze Engineers control box |
TDT RZ6 Multi-I/O high-frequency auditory processor |
Speaker |
Real-time sound lever reader |
Electronic controller and sound generator |
ABR recordings involve placing electrodes near the subject’s scalp to capture evoked potentials. When sound stimuli are presented, the auditory brainstem pathways—including cochlear ganglion neurons and their associated fiber tracts and nuclei—generate electrical responses that provide insights into the health of these neural structures. The ABR waveform typically comprises five distinct components, labeled I to V, reflecting the activation of the auditory nerve and the processing of sound through the brainstem pathways.
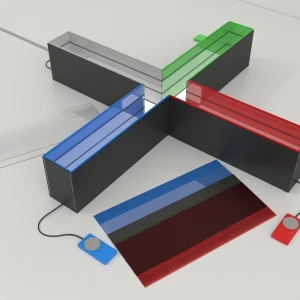
The Rodent Audible Brainstem Response (ABR) Test setup features a soundproof isolation chamber with an animal enclosure, equipped with LED and infrared lighting. An external device can be connected through a customizable device slot, and a camera mount is positioned at the top of the chamber for observation.
The Maze Engineers control box interfaces with the TDT RZ6 processor to deliver TTL signals that synchronize with each stimulus presentation.
Sound stimuli are produced by a generator with adjustable parameters, and are played through two speakers capable of emitting tones or white noise. A sound meter is used to monitor and ensure accurate sound levels.
The rodent’s auditory neural pathway generates electrical responses to these stimuli, which the TDT RZ6 then records in high fidelity, capturing neural activity, stimulus waveforms, and related behavioral events.
The Conduct ABR Maze software enables the creation of customized sound stimuli protocols, allowing adjustments to parameters such as frequency, dB level, phase, and duration.
Follow appropriate laboratory protocols, surgical hygiene, and animal welfare practices before commencing. Clean and sterilize all surgical equipment and other apparatus.
The following protocol considers a mouse as the subject. The protocol can be applied to other small rodents as well.
These data recored from the rodent auditory brainstem response apparatus can be used to evaluate hearing function, diagnose hearing disorders, study the effects of ototoxic drugs or noise exposure on the auditory system, assess the efficacy of hearing protection devices or therapies, and investigate the neural mechanisms of hearing and auditory processing.
| Rodent Auditory Brainstem Response Test | Brain response recorder, Conduct ABR software, Maze Engineers Sound System, Mouse Auditory Brainstem Response Test |
|---|
There are no questions yet. Be the first to ask a question about this product.
Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.